
You are now leaving NuplazidHCP.com to visit a website that is not owned or operated by Acadia Pharmaceuticals Inc. Links to all outside sites are provided as resources only. Acadia Pharmaceuticals Inc. does not accept responsibility or liability for the content or services of other websites.

You are now leaving NuplazidHCP.com to visit a website that is not owned or operated by Acadia Pharmaceuticals Inc. Links to all outside sites are provided as resources only. Acadia Pharmaceuticals Inc. does not accept responsibility or liability for the content or services of other websites.
NUPLAZID® (pimavanserin) is indicated for the treatment of hallucinations and delusions associated with Parkinson’s disease (PD) psychosis.
NUPLAZID Safety Data
Adverse drug reactions reported in ≥2% and greater than placebo in 6-week
placebo-controlled studies1



No differences in safety were reported based on age, gender, or MMSE score (MMSE 21-24 vs ≥25)1
- 80% of patients on NUPLAZID were aged 65 years or older (Maximum age on NUPLAZID vs placebo was 85 and 90, respectively)1,2
Low rates of discontinuation due to adverse reactions
8% (n=16) of patients treated with NUPLAZID discontinued due to adverse reactions vs 4% (n=10) with placebo.1
-
The adverse reactions that occurred in more than one patient and with an incidence of at least twice that of placebo were hallucination (2% NUPLAZID vs <1% placebo), urinary tract infection (1% NUPLAZID vs <1% placebo), and fatigue (1% NUPLAZID vs 0% placebo)
Important Safety Information
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
-
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
-
NUPLAZID is not approved for the treatment of patients with dementia who experience psychosis unless their hallucinations and delusions are related to Parkinson’s disease.
Sedation-related and orthostatic hypotension–related events in 6-week placebo-controlled studies2



-
There are no warnings or precautions in the Prescribing Information for sedation or orthostatic hypotension1
-
Sedation-related events included sedation, somnolence, fatigue, asthenia, lethargy, and hypersomnia2
-
Orthostatic hypotension–related events included dizziness, hypotension, orthostatic hypotension, orthostatic intolerance, syncope, vertigo positional, postural orthostatic tachycardia syndrome, and vertigo2
Important Safety Information
Warnings and Precautions: QT Interval Prolongation
-
NUPLAZID prolongs the QT interval. The use of NUPLAZID should be avoided in patients with known QT prolongation or in combination with other drugs known to prolong QT interval (e.g., Class 1A antiarrhythmics, Class 3 antiarrhythmics, certain antipsychotics or antibiotics).
-
NUPLAZID should also be avoided in patients with a history of cardiac arrhythmias, as well as other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death, including symptomatic bradycardia, hypokalemia or hypomagnesemia, and presence of congenital prolongation of the QT interval.
Drug Interactions:
-
Coadministration with strong CYP3A4 inhibitors increases NUPLAZID exposure. Reduce NUPLAZID dose to 10 mg taken orally as one tablet once daily.
-
Coadministration with strong or moderate CYP3A4 inducers reduces NUPLAZID exposure. Avoid concomitant use of strong or moderate CYP3A4 inducers with NUPLAZID.
Long-term safety findings from an open-label safety study
More than half of enrolled patients remained in the open-label safety extension study for 1 year3,4



No new or unexpected safety findings were observed in patients receiving NUPLAZID in an OLE study3
Patient discontinuation and treatment-emergent adverse events in open-label extension safety study over 9 years of study follow‑up
| Primary reason | Overall, n (%) |
|---|---|
| Voluntary withdrawal of consent | 167 (36.4) |
| Adverse event | 88 (19.2) |
| Sponsor decision | 55 (12.0) |
| Disease progression | 46 (10.0) |
†Other reasons for termination (<10%) included death (n=34), investigator decision, subject non-compliance, and lost to follow-up.‡
‡Over 9 years of study follow-up, the total number of all-cause deaths was 59 patients. The deaths occurred during treatment or within 30 days after the last dose of NUPLAZID.
The types of adverse events reported in an OLE study were comparable to the 6-week placebo-controlled studies3
| Adverse event | Number (%) of patients |
|---|---|
| Fall | 147 (32.0) |
| Urinary tract infection | 87 (19.0) |
| Hallucination | 63 (13.7) |
| Weight decreased | 57 (12.4) |
| Confusional state | 51 (11.1) |
| Constipation | 47 (10.2) |
Important note: The results of this OLE safety study should be interpreted with the following limitations: open-label design, lack of control arm, inability to assess patients long-term after discontinuation, and high discontinuation rate due to various reasons over >4 years of the study duration. This could represent chance findings.
Maintenance safety data: Tolerability in patients with PD psychosis with PD dementia
Below are the safety and tolerability findings from a post hoc subgroup analysis of patients with PD psychosis with PD dementia from the Phase 3, double-blind, placebo-controlled, randomized discontinuation study. The study included a 12-week, open-label phase. Patients who met the open-label response criteria* entered the 26-week, double-blind, randomized phase and were evaluated for relapse of psychosis. The study was stopped early for demonstrating positive efficacy at a preplanned Interim Analysis.
Open-label phase: Treatment-emergent adverse events reported in ≥ 3%5†
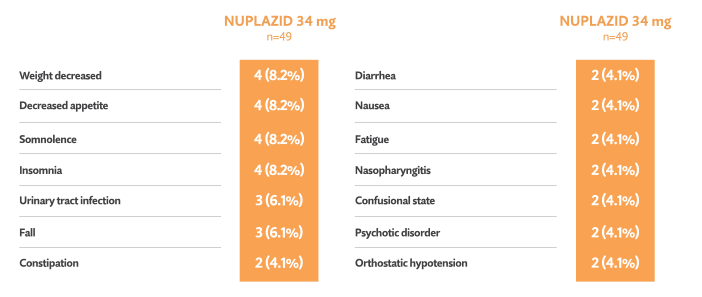


- One death occurred during the open-label phase (considered unrelated to trial drug by investigator)5
- 7/49 patients (14.3%) discontinued in the open-label phase due to adverse events5
Double-blind phase: Treatment-emergent events reported in ≥3% in NUPLAZID-treated patients and greater than placebo5†
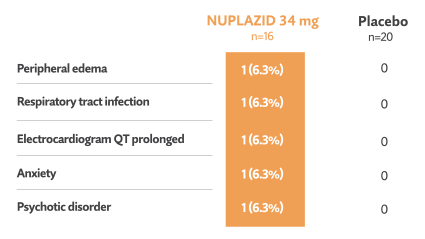


- No deaths occurred during the double-blind phase in this subgroup5
- 1/16 patients (6.3%) treated with NUPLAZID and 2/20 patients (10%) taking placebo discontinued in the double-blind phase due to adverse events5
No overall change in mean MMSE over 26 weeks5†‡
Impact on cognitive function was assessed by the MMSE scale.5 A decrease in score indicates impairment.6
- Double-blind baseline MMSE total score, mean (SE): NUPLAZID, 19.6 (1.26); placebo, 19.3 (1.29)5
- Double-blind phase: Mean (SE) MMSE score change was 0.8 (0.75), n=4 in patients taking NUPLAZID 34 mg vs 0.5 (2.50), n=2 with placebo over 26 weeks. No difference was observed between patients treated with NUPLAZID 34 mg and placebo-treated patients5
No overall change in mean ESRS-A over 26 weeks5†‡
Extrapyramidal symptoms (EPS) were assessed by the ESRS-A scale.5 A higher score indicates the onset or worsening of EPS.7
- Double-blind baseline ESRS-A total score, mean (SE): NUPLAZID, 27.4 (3.99); placebo, 26.3 (3.14)5
- Double-blind phase: Mean (SE) ESRS-A score change was 1.0 (2.68), n=4 in patients taking NUPLAZID 34 mg vs 0.5 (1.50), n=2 with placebo over 26 weeks. No difference was observed between patients treated with NUPLAZID 34 mg and placebo-treated patients5
Important considerations
- The results of the post hoc subgroup analysis presented here are descriptive and supportive of the clinical information related to the FDA-approved indication for the treatment of hallucinations and delusions associated with PD psychosis
- These results should be interpreted cautiously since the study was not designed or powered to demonstrate an effect in the subgroup
*Response was defined as a ≥30% reduction (improvement) in SAPS-H+D total score and a CGI-I score of 1 (very much improved) or 2 (much improved), relative to open-label baseline, at both Weeks 8 and 12.5
†PD psychosis with PD dementia NUPLAZID 34 mg subgroup as of October 30, 2019 (study end date).5
‡Based on patients remaining in the study by the study end date.5
ESRS-A=Extrapyramidal Symptoms Rating Scale-Abbreviated; MMSE=Mini Mental State Examination; SE=standard error.
Important Safety Information
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
-
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
-
NUPLAZID is not approved for the treatment of patients with dementia who experience psychosis unless their hallucinations and delusions are related to Parkinson’s disease.
Warnings and Precautions: QT Interval Prolongation
- NUPLAZID prolongs the QT interval. The use of NUPLAZID should be avoided in patients with known QT prolongation or in combination with other drugs known to prolong QT interval (e.g., Class 1A antiarrhythmics, Class 3 antiarrhythmics, certain antipsychotics or antibiotics).
- NUPLAZID should also be avoided in patients with a history of cardiac arrhythmias, as well as other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death, including symptomatic bradycardia, hypokalemia or hypomagnesemia, and presence of congenital prolongation of the QT interval.
6-week Safety & Tolerability
Adverse drug reactions reported in ≥2% and greater than placebo in 6-week
placebo-controlled studies1



No differences in safety were reported based on age, gender, or MMSE score (MMSE 21-24 vs ≥25)1
- 80% of patients on NUPLAZID were aged 65 years or older (maximum age on NUPLAZID vs placebo was 85 and 90, respectively)1
Low rates of discontinuation due to adverse reactions
8% (n=16) of patients treated with NUPLAZID discontinued due to adverse reactions vs 4% (n=10) with placebo.1
-
The adverse reactions that occurred in more than one patient and with an incidence of at least twice that of placebo were hallucination (2% NUPLAZID vs <1% placebo), urinary tract infection (1% NUPLAZID vs <1% placebo), and fatigue (1% NUPLAZID vs 0% placebo)
Important Safety Information
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
-
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
-
NUPLAZID is not approved for the treatment of patients with dementia who experience psychosis unless their hallucinations and delusions are related to Parkinson’s disease.
Sedation-related and orthostatic hypotension–related events in 6-week placebo-controlled studies2



-
There are no warnings or precautions in the Prescribing Information for sedation or orthostatic hypotension1
-
Sedation-related events included sedation, somnolence, fatigue, asthenia, lethargy, and hypersomnia2
-
Orthostatic hypotension–related events included dizziness, hypotension, orthostatic hypotension, orthostatic intolerance, syncope, vertigo positional, postural orthostatic tachycardia syndrome, and vertigo2
Important Safety Information
Warnings and Precautions: QT Interval Prolongation
-
NUPLAZID prolongs the QT interval. The use of NUPLAZID should be avoided in patients with known QT prolongation or in combination with other drugs known to prolong QT interval (e.g., Class 1A antiarrhythmics, Class 3 antiarrhythmics, certain antipsychotics or antibiotics).
-
NUPLAZID should also be avoided in patients with a history of cardiac arrhythmias, as well as other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death, including symptomatic bradycardia, hypokalemia or hypomagnesemia, and presence of congenital prolongation of the QT interval.
Drug Interactions:
-
Coadministration with strong CYP3A4 inhibitors increases NUPLAZID exposure. Reduce NUPLAZID dose to 10 mg taken orally as one tablet once daily.
-
Coadministration with strong or moderate CYP3A4 inducers reduces NUPLAZID exposure. Avoid concomitant use of strong or moderate CYP3A4 inducers with NUPLAZID.
Long-term Safety Findings
Long-term safety findings from an open-label safety study
More than half of enrolled patients remained in the open-label safety extension study for 1 year3,4



No new or unexpected safety findings were observed in patients receiving NUPLAZID in an OLE study3
Patient discontinuation and treatment-emergent adverse events in open-label extension safety study over 9 years of study follow‑up
| Primary reason | Overall, n (%) |
|---|---|
| Voluntary withdrawal of consent | 167 (36.4) |
| Adverse event | 88 (19.2) |
| Sponsor decision | 55 (12.0) |
| Disease progression | 46 (10.0) |
†Other reasons for termination (<10%) included death (n=34), investigator decision, subject non-compliance, and lost to follow-up.‡
‡Over 9 years of study follow-up, the total number of all-cause deaths was 59 patients. The deaths occurred during treatment or within 30 days after the last dose of NUPLAZID.
The types of adverse events reported in an OLE study were comparable to the 6-week placebo-controlled studies3
| Adverse event | Number (%) of patients |
|---|---|
| Fall | 147 (32.0) |
| Urinary tract infection | 87 (19.0) |
| Hallucination | 63 (13.7) |
| Weight decreased | 57 (12.4) |
| Confusional state | 51 (11.1) |
| Constipation | 47 (10.2) |
Important note: The results of this OLE safety study should be interpreted with the following limitations: open-label design, lack of control arm, inability to assess patients long-term after discontinuation, and high discontinuation rate due to various reasons over >4 years of the study duration. This could represent chance findings.
Maintenance Safety Data (post hoc analysis)
Maintenance safety data: Tolerability in patients with PD psychosis with PD dementia
Below are the safety and tolerability findings from a post hoc subgroup analysis of patients with PD psychosis with PD dementia from the Phase 3, double-blind, placebo-controlled, randomized discontinuation study. The study included a 12-week, open-label phase. Patients who met the open-label response criteria* entered the 26-week, double-blind, randomized phase and were evaluated for relapse of psychosis. The study was stopped early for demonstrating positive efficacy at a preplanned Interim Analysis.
Open-label phase: Treatment-emergent adverse events reported in ≥ 3%5†



- One death occurred during the open-label phase (considered unrelated to trial drug by investigator)5
- 7/49 patients (14.3%) discontinued in the open-label phase due to adverse events5
Double-blind phase: Treatment-emergent events reported in ≥3% in NUPLAZID-treated patients and greater than placebo5†



- No deaths occurred during the double-blind phase in this subgroup5
- 1/16 patients (6.3%) treated with NUPLAZID and 2/20 patients (10%) taking placebo discontinued in the double-blind phase due to adverse events5
No overall change in mean MMSE over 26 weeks5†‡
Impact on cognitive function was assessed by the MMSE scale.5 A decrease in score indicates impairment.6
- Double-blind baseline MMSE total score, mean (SE): NUPLAZID, 19.6 (1.26); placebo, 19.3 (1.29)5
- Double-blind phase: Mean (SE) MMSE score change was 0.8 (0.75), n=4 in patients taking NUPLAZID 34 mg vs 0.5 (2.50), n=2 with placebo over 26 weeks. No difference was observed between patients treated with NUPLAZID 34 mg and placebo-treated patients5
No overall change in mean ESRS-A over 26 weeks5†‡
Extrapyramidal symptoms (EPS) were assessed by the ESRS-A scale.5 A higher score indicates the onset or worsening of EPS.7
- Double-blind baseline ESRS-A total score, mean (SE): NUPLAZID, 27.4 (3.99); placebo, 26.3 (3.14)5
- Double-blind phase: Mean (SE) ESRS-A score change was 1.0 (2.68), n=4 in patients taking NUPLAZID 34 mg vs 0.5 (1.50), n=2 with placebo over 26 weeks. No difference was observed between patients treated with NUPLAZID 34 mg and placebo-treated patients5
Important considerations
- The results of the post hoc subgroup analysis presented here are descriptive and supportive of the clinical information related to the FDA-approved indication for the treatment of hallucinations and delusions associated with PD psychosis
- These results should be interpreted cautiously since the study was not designed or powered to demonstrate an effect in the subgroup
*Response was defined as a ≥30% reduction (improvement) in SAPS-H+D total score and a CGI-I score of 1 (very much improved) or 2 (much improved), relative to open-label baseline, at both Weeks 8 and 12.5
†PD psychosis with PD dementia NUPLAZID 34 mg subgroup as of October 30, 2019 (study end date).5
‡Based on patients remaining in the study by the study end date.5
ESRS-A=Extrapyramidal Symptoms Rating Scale-Abbreviated; MMSE=Mini Mental State Examination; SE=standard error.
Important Safety Information
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
-
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
-
NUPLAZID is not approved for the treatment of patients with dementia who experience psychosis unless their hallucinations and delusions are related to Parkinson’s disease.
Warnings and Precautions: QT Interval Prolongation
- NUPLAZID prolongs the QT interval. The use of NUPLAZID should be avoided in patients with known QT prolongation or in combination with other drugs known to prolong QT interval (e.g., Class 1A antiarrhythmics, Class 3 antiarrhythmics, certain antipsychotics or antibiotics).
- NUPLAZID should also be avoided in patients with a history of cardiac arrhythmias, as well as other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death, including symptomatic bradycardia, hypokalemia or hypomagnesemia, and presence of congenital prolongation of the QT interval.
Learn about the proven efficacy of NUPLAZID.
Acadia Connect® provides comprehensive prescription support.
 IMPORTANT SAFETY INFORMATION and INDICATION
IMPORTANT SAFETY INFORMATION and INDICATION
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
- Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
- NUPLAZID is not approved for the treatment of patients with dementia who experience psychosis unless their hallucinations and delusions are related to Parkinson’s disease.
- Contraindication: NUPLAZID is contraindicated in patients with a history of a hypersensitivity reaction to pimavanserin or any of its components. Rash, urticaria, and reactions consistent with angioedema (e.g., tongue swelling, circumoral edema, throat tightness, and dyspnea) have been reported.
-
Warnings and Precautions: QT Interval Prolongation
-
NUPLAZID prolongs the QT interval. The use of NUPLAZID should be avoided in patients with known QT prolongation or in combination with other drugs known to prolong QT interval (e.g., Class 1A antiarrhythmics, Class 3 antiarrhythmics, certain antipsychotics or antibiotics).
-
NUPLAZID should also be avoided in patients with a history of cardiac arrhythmias, as well as other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death, including symptomatic bradycardia, hypokalemia or hypomagnesemia, and presence of congenital prolongation of the QT interval.
-
- Adverse Reactions: The adverse reactions (≥2% for NUPLAZID and greater than placebo) were peripheral edema (7% vs 2%), nausea (7% vs 4%), confusional state (6% vs 3%), hallucination (5% vs 3%), constipation (4% vs 3%), and gait disturbance (2% vs <1%).
-
Drug Interactions:
-
Coadministration with strong CYP3A4 inhibitors increases NUPLAZID exposure. Reduce NUPLAZID dose to 10 mg taken orally as one tablet once daily.
-
Coadministration with strong or moderate CYP3A4 inducers reduces NUPLAZID exposure. Avoid concomitant use of strong or moderate CYP3A4 inducers with NUPLAZID.
-
Indication NUPLAZID is indicated for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis.
Dosage and Administration Recommended dose: 34 mg capsule taken orally once daily, without titration, with or without food.
NUPLAZID is available as 34 mg capsules and 10 mg tablets.
Please read the full Prescribing Information, including Boxed WARNING.
References:
- Acadia Pharmaceuticals Inc. NUPLAZID® [package insert]. San Diego, CA; 2023.
- Acadia Pharmaceuticals Inc. NUPLAZID Advisory Committee Briefing Document. San Diego, CA: Sponsor Background Information for a Meeting of the Psychopharmacologic Drugs Advisory Committee. March 29, 2016.
- Ballard CG, Kreitzman DL, Isaacson S, et al. Long-term evaluation of open-label pimavanserin safety and tolerability in Parkinson’s disease psychosis. Parkinsonism Relat Disord. 2020;77:100-106.
- Acadia Pharmaceuticals Inc. Data on file. ACP-103-015. KM estimates in percent of subjects in treatment over time. 2018.
- Data on file. ACP-103-045_PDD 34 mg subgroup_Sep2021.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
- Chouinard G, Margolese HC. Manual for the Extrapyramidal Symptom Rating Scale (ESRS). Schizophr Res. 2005;76(2-3):247-265.